PEDOT:PSS Microparticles for Extrudable and Bioencapsulating Conducting Granular Hydrogel Bioelectronics
PEDOT:PSS Microparticles for Extrudable and Bioencapsulating Conducting Granular Hydrogel Bioelectronics
Goestenkors, A. P.; Yu, J. S.; Park, J.; Wu, Y.; Espinoza, C. J. V.; Friedman, L. C.; Okafor, S. S.; Liu, T.; Chatterjee, S.; Debnath, A.; Semar, B. A.; O'Hare, C. P.; Alvarez, R. M.; Singamaneni, S.; Raman, B.; Rutz, A. L.
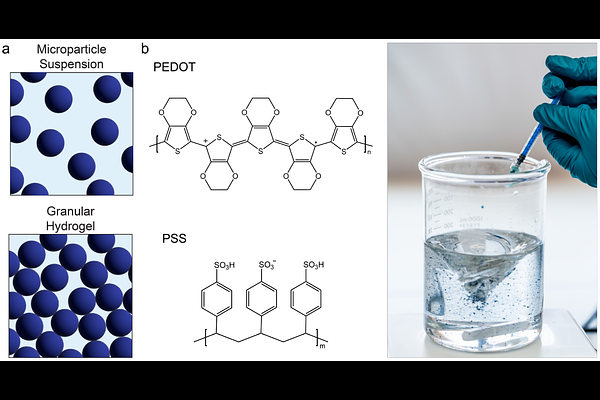
AbstractConducting hydrogels are promising materials for forming physiomimetic bioelectronic interfaces to monitor and stimulate biological activity. However, most developed materials are non-microporous and possess fixed shapes, both of which can limit the integration of cells and tissues with devices. In non-conducting biomaterials, materials fabrication strategies imparting microporosity and dynamic mechanical properties have been shown to support cell infiltration and support biointerfaces of various geometries. Specifically, granular hydrogels have enabled encapsulating, conformal, and injectable interfaces through these features. However, granular hydrogels remain largely unexplored as conducting biomaterials. We present methods for fabricating spherical, poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) hydrogel microparticles. When densely packed, these microparticles form a conducting granular hydrogel with microporosity as well as shear-thinning and self-healing dynamic mechanical properties. The PEDOT:PSS granular hydrogel can be extruded and maintain structure post-3D printing. Modulating microparticle PSS content achieves high granular hydrogel conductivity (137 S/m), and microparticles exhibit excellent cytocompatibility (>98% viability). Finally, we demonstrate utility as bioencapsulating electrodes for electrophysiological monitoring. These results highlight the functionality of our PEDOT:PSS conducting granular hydrogel, suggesting its potential as 3D printed bioencapsulating electrodes, 3D tissue engineering scaffolds for monitoring encapsulated cells, and injectable therapies for enhanced cell recruitment and tissue regeneration combined with electronic stimulation.