Rab27b promotes lysosomal function and alpha-synuclein clearance in neurons
Rab27b promotes lysosomal function and alpha-synuclein clearance in neurons
Scholz, K.; Pattanayak, R.; Roschonporn, E.; Pair, F. S.; Nobles, A.; Yacoubian, T. A.
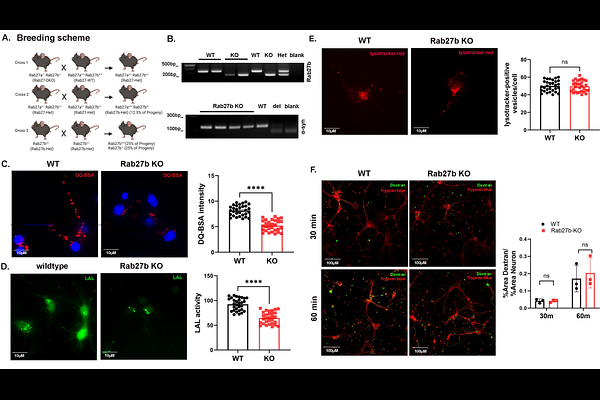
AbstractAlpha-synuclein is the key pathogenic protein implicated in synucleinopathies including Parkinson\'s Disease (PD) and Dementia with Lewy Bodies (DLB). In these diseases, alpha-synuclein is thought to spread between cells where it accumulates and induces pathology; however, mechanisms that drive its propagation or aggregation are poorly understood. We have previously reported that the small GTPase Rab27b is elevated in human PD and DLB and that it can mediate the autophagic clearance and toxicity of alpha-synuclein in a paracrine alpha-synuclein cell culture neuronal model. Here, we expanded our previous work and further characterized a role for Rab27b in neuronal lysosomal processing and alpha-synuclein clearance. We found that Rab27b KD in this alpha-synuclein inducible neuronal model resulted in lysosomal dysfunction and increased alpha-synuclein levels in lysosomes. Similar lysosomal proteolytic defects and enzymatic dysfunction were observed in both primary neuronal cultures and brain lysates from Rab27b knock-out (KO) mice. Alpha-synuclein aggregation was exacerbated in Rab27b KO neurons upon treatment with alpha-synuclein preformed fibrils. We found no changes in lysosomal counts or lysosomal pH in either model, but we did identify defects in acidic vesicle trafficking in Rab27b KO primary neurons which may drive lysosomal dysfunction and promote alpha-synuclein aggregation. Rab27b overexpression enhanced lysosomal activity and reduced insoluble alpha-synuclein accumulation. Finally we found elevated Rab27b levels in human postmortem incidental Lewy Body Disease (iLBD) subjects relative to healthy controls. These data suggest a role for Rab27b in neuronal lysosomal activity and identify it as a potential therapeutic target in synucleinopathies.