High-throughput thermodynamic and kinetic measurements of transcription factor/DNA mutations reveal how conformational heterogeneity can shape motif selectivity
High-throughput thermodynamic and kinetic measurements of transcription factor/DNA mutations reveal how conformational heterogeneity can shape motif selectivity
Fordyce, P. M.; Hastings, R.; Aditham, A.; DelRosso, N.; Suzuki, P.
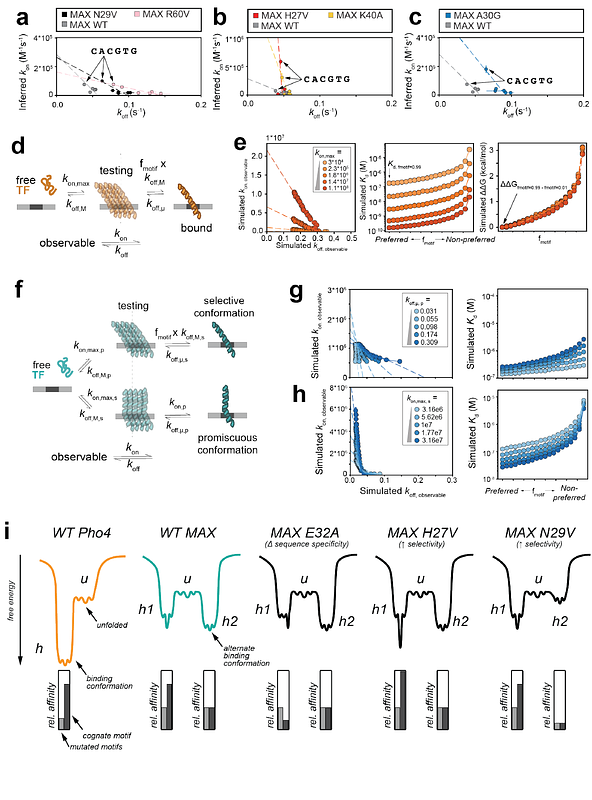
AbstractTranscription factors (TFs) bind DNA sequences with a range of affinities, yet the mechanisms determining energetic differences between high- and low-affinity sequences (\"selectivity\") remain poorly understood. Here, we investigated two basic helix-loop-helix TFs, MAX (H. sapiens) and Pho4 (S. cerevisiae), that bind the same high-affinity sequence with highly similar nucleotide-contacting residues and bound structures but are differentially selective for non-cognate sequences. By measuring >1700 Kds and >500 rate constants for Pho4 and MAX mutant libraries binding multiple DNA sequences and comparing these measurements with thermodynamic and kinetic models, we identify the biophysical mechanisms by which changes to TF sequence alter both bound and unbound conformational ensembles to shape specificity landscapes. These results highlight the importance of conformational heterogeneity in determining sequence specificity and selectivity and can guide future efforts to engineer nucleic acid-binding proteins with enhanced selectivity.