Efficient coding explains neural response homeostasis and stimulus-specific adaptation
Efficient coding explains neural response homeostasis and stimulus-specific adaptation
Young, E. J.; Ahmadian, Y.
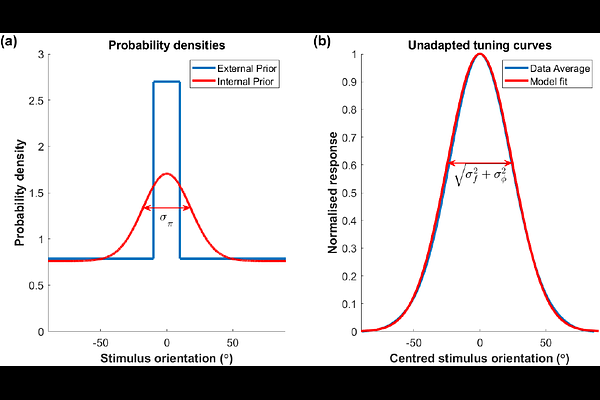
AbstractNeurons are typically sensitive to a small fraction of stimulus space. If the environment changes, making certain stimuli more prevalent, neurons sensitive to those stimuli would respond more often and therefore, if the stimulus-response mapping remains fixed, have a higher average firing rate. However, sufficiently prolonged exposure to the new environment typically causes such neurons to adapt by responding less vigorously. If adaptation consistently returns the average firing rate of neurons, or populations of similarly tuned neurons, to its value prior to environmental shift, it is termed firing rate homeostasis. In sensory cortex, adaptation is typically also stimulus specific, with neurons maintaining or even increasing their responsiveness to stimuli far from over-represented ones. Here, we present a normative explanation of firing rate homeostasis grounded in the efficient coding principle. Unlike previous theories based on efficient coding, we formulate the problem in a computation-agnostic manner, enabling our framework to apply far from the sensory periphery. We show that homeostasis can provide an optimal solution to a trade-off between coding fidelity and the metabolic cost of neural firing. We provide quantitative conditions necessary for the optimality of firing rate homeostasis, and predict how adaptation should deviate from homeostasis when these conditions are violated. Based on biological estimates of relevant parameters, we show that these conditions do hold in areas of cortex where homeostatic adaptation has been observed. Finally, we apply our framework to distributed distributional codes, a specific computational theory of neural representations serving Bayesian inference. We show that the resultant coding scheme can be accomplished by divisive normalisation with adaptive weights. We further demonstrate how homeostatic coding, coupled with such Bayesian neural representations, explains stimulus-specific adaptation, as observed, e.g., in the primary visual cortex.