Curvature-dependent morphological reorganization of the endoplasmic reticulum determines the mode of epithelial migration
Curvature-dependent morphological reorganization of the endoplasmic reticulum determines the mode of epithelial migration
Rawal, S.; Keshavanarayana, P.; Manoj, D.; Khuntia, P.; Banerjee, S.; Thurakkal, B.; Marwaha, R.; Spill, F.; Das, T.
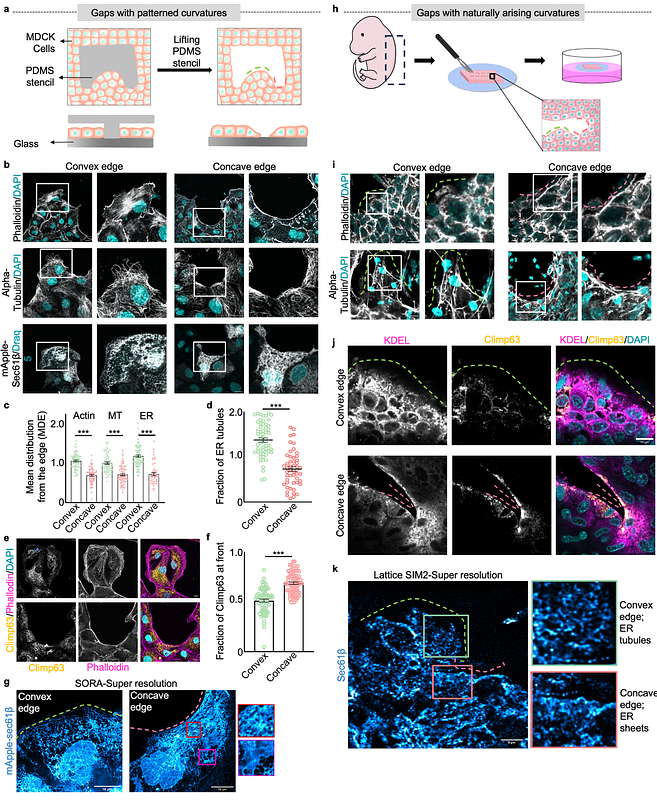
AbstractFrom single-cell extrusion to centimeter-sized wounds, epithelial gaps of various sizes and geometries appear in all organisms. For gap closure, epithelial cells invoke two orthogonal modes: lamellipodial crawling at the convex edge and purse-string-like movements at the concave edge. The mechanisms underlying these differential responses to geometric cues remain elusive. Here we perform an intracellular cartography to reveal that in both micropatterned and naturally arising gaps, the endoplasmic reticulum (ER) undergoes edge curvature-dependent morphological reorganizations with convex and concave edges promoting ER tubules and sheets, respectively. This reorganization depends on cytoskeleton-generated protrusive and contractile forces. Additionally, theoretical modeling predicts that the curvature-specific ER morphology leads to a lower strain energy state. ER tubules at the convex edge favor perpendicularly oriented focal adhesions, supporting lamellipodial crawling while ER sheets at the concave edge favor parallelly oriented focal adhesions, supporting purse-string-like movements. Altogether, ER emerges as a central player in cellular mechanotransduction, which orchestrates two orthogonal modes of cell migration by integrating signals from cytoskeletal networks.