EPLINα controls integrin recycling from Rab21 endosomes to drive breast cancer cell migration
EPLINα controls integrin recycling from Rab21 endosomes to drive breast cancer cell migration
Jäntti, N. Z.; Moreno-Layseca, P.; Chastney, M. R.; Dibus, M.; Conway, J. R. W.; Leppänen, V.-M.; Hamidi, H.; Eylmann, K.; Oliveira-Ferrer, L.; Veltel, S.; Ivaska, J.
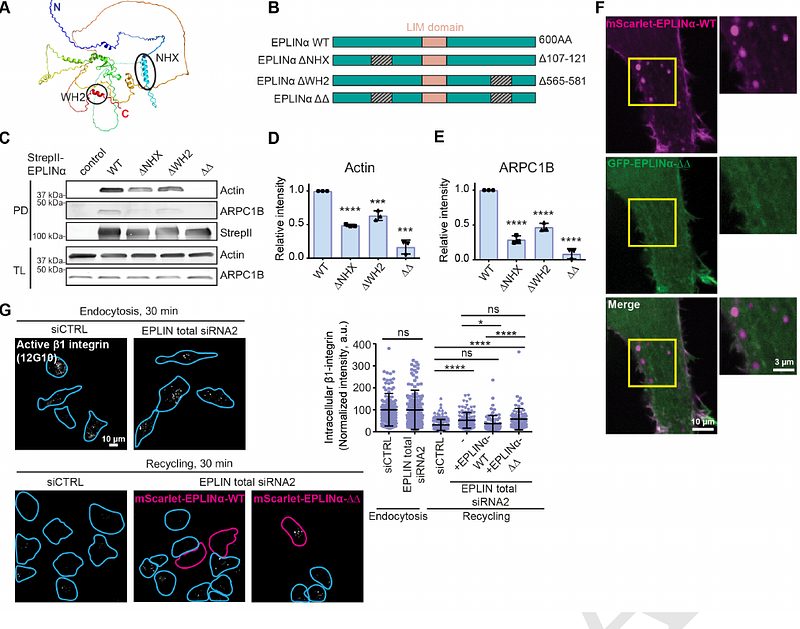
AbstractEPLIN, an actin-binding protein, has been described as both a tumour promoter and tumour suppressor in different cancers. EPLIN isoform( or {beta})-specific functions, which remain largely unknown, could explain these opposing roles. We observed distinct EPLIN-isoform localization; EPLIN is recruited to actin in plasma membrane ruffles and endosomes, while EPLIN{beta} resides on actin stress fibers. We identified two EPLIN actin-binding regions and demonstrated EPLIN interaction with Rab21, an established regulator of {beta}1-integrin endosomal traffic. EPLIN co-localizes with Rab21 and F-actin on recycling endosomes in an actin binding-dependent manner and supports {beta}1-integrin recycling and cell migration. Using BioID, we identified coronin 1C as an EPLIN proximal protein, which localizes at Rab21-containing endosomes in an EPLIN-dependent manner. EPLIN expression was linked to increased breast cancer cell motility, and high EPLIN-to-EPLIN{beta} ratio correlated with a mesenchymal phenotype in patient samples. Our work unveils unprecedented EPLIN isoform-specific functions relevant to breast cancer and beyond.


