Deazaguanylation is a nucleobase-protein conjugation required for type IV CBASS immunity
Deazaguanylation is a nucleobase-protein conjugation required for type IV CBASS immunity
Wassarman, D. R.; Pfaff, P.; Paulo, J. A.; Gygi, S. P.; Shokat, K. M.; Kranzusch, P. J.
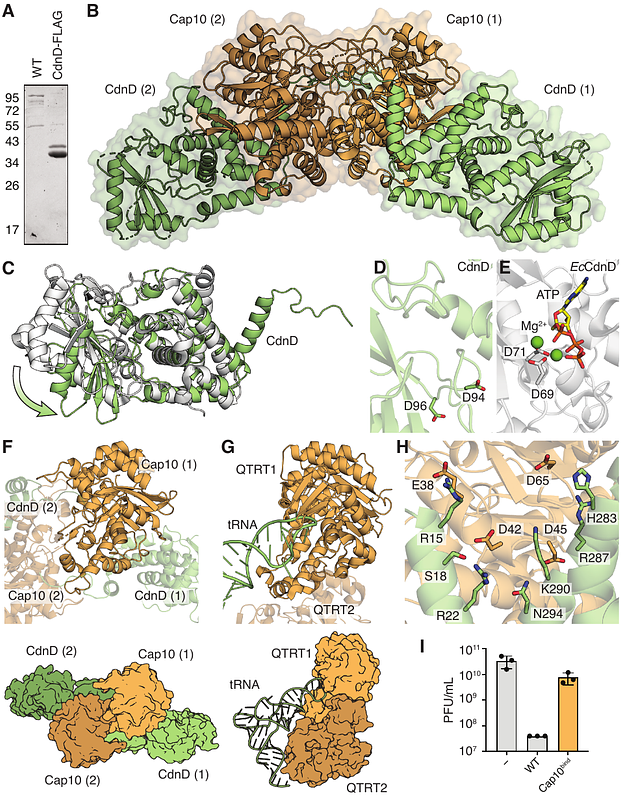
Abstract7-deazapurines are nucleobase analogs essential for nucleic acid modifications in nearly all cellular life. Here, we discover a role for 7-deazapurines in protein modification within type IV CBASS anti-phage defense and define functions for CBASS ancillary proteins Cap9 and Cap10 in nucleobase-protein conjugation. A structure of Cap10 reveals a tRNA transglycosylase-family enzyme remodeled to bind the modified N-terminus of a partner cGAS/DncV-like nucleotidyltransferase linked to a 7-amido-7-deazaguanine (NDG) nucleobase. The structure of Cap9 explains how this QueC-like enzyme co-opts a 7-deazapurine biosynthetic reaction mechanism for NDG conjugation. We demonstrate that Cap9, Cap10, and NDG conjugation are essential for host defense against phage infection. Our results define a previously unknown 7-deazapurine protein modification and explain how nucleobase biosynthetic machinery has been repurposed for antiviral immunity.